Our Technology
Transcranial Electromagnetic Treatment leveraging Radio Frequencies (TEMT-RF)
Propagating electromagnetic waves, showing their inter-digitated electrical (red) and magnetic (blue) components.
NeuroEM’s Co-Founder and Scientist Emeritus, Dr. Gary Arendash, was a Research Professor at the University of South Florida (USF) and the Florida Alzheimer’s Disease Research Center when he began investigating the effects of Transcranial Electromagnetic Treatment leveraging Radio Frequencies (TEMT-RF) on brain pathology and cognitive function in Alzheimer’s transgenic mice with his USF colleague, Dr. Chuanhai Cao.
Alzheimer’s disease appears to be initiated by the aggregation of an abnormal brain protein called β-amyloid (Aβ) during aging. Alzheimer’s transgenic mice are genetically engineered so that their neurons have the same human gene that produces human Aβ. Once produced, this human Aβ protein then aggregates inside and outside neurons in their brains to start the Alzheimer’s disease process, just as in humans.
In such Alzheimer’s mice, it was found that TEMT-RF begun early in adulthood (before Alzheimer’s neuropathology and cognitive impairment occurs) protected Alzheimer’s transgenic mice from otherwise certain cognitive impairment. If TEMT-RF was delayed until old age (when extensive neuropathology and cognitive impairment were present), TEMT-RF reversed both the Aβ protein aggregation and the cognitive impairment of these old Alzheimer’s transgenic mice. This anti-Aβ aggregating effect of TEMT-RF occurred both outside of neurons (reducing the size of large Aβ deposits) and inside neurons (reducing small Aβ oligomers).
Growing evidence indicates that these small Aβ oligomers inside neurons are the primary initiators of neuronal dysfunction and death in Alzheimer’s. Importantly, no deleterious effects of daily TEMT-RF (for up to 8 months) were evident in the brain or body of treated Alzheimer’s mice.
Following their initial 2010 paper, Dr. Arendash and colleagues published five additional pre-clinical papers that further underscore the utility of TEMT-RF in Alzheimer’s transgenic mice to enhance cognitive function and reduce brain Alzheimer’s neuropathology. These follow-up papers identified two additional mechanisms of action that work in concert with the anti-Aβ aggregation mechanism initially identified – namely, mitochondrial enhancement (to increase energy production in neurons) and increased neuronal activity in brain regions impacted by Alzheimer’s.
These additional mechanisms are important because brain mitochondrial dysfunction and decreased neuronal activity occur very early in the Alzheimer’s process – before memory impairment is noticed.
NeuroEM Therapeutics’ most recent basic science research (supported by NIH SBIR grant 1R43NS90653-01A1) has indicated the ability of TEMT-RF to not only prevent Aβ aggregation but to also prevent p-tau aggregation in human Alzheimer’s brain tissue (see below figure; purple cylinders represent either Aβ or p-tau).
This is significant because Aβ aggregation induces p-tau aggregation, both of which then appear to cause the neuronal dysfunction and death of Alzheimer’s. Thus, TEMT-RF dissociates both of the toxic proteins currently thought to be at the root cause of Alzheimer’s. To our knowledge, no drug currently being developed has this critical ability.
TEMT-RF can block or reverse aggregation of both Aβ and p-tau.
Mounting evidence indicates that disaggregation of Aβ and p-tau oligomers by TEMT-RF occurs through destabilization of the relatively weak hydrogen bonds between oligomer monomers through dipole-dipole interactions, vibration, and/or resonance phenomena. In this regard, electromagnetic/radiofrequency waves in the range used in our studies have been shown to cause reduced dipole-dipole interactions (dielectric loss), which leads to a decrease in inter-molecular hydrogen bonding. Indeed, the toxic protein β-sheet aggregates of Aβ and tau have a common backbone polarization that is stabilized via two-electron interactions of hydrogen bonds – a backbone that appears to be selectively disrupted by the radiofrequency waves we utilized
TEMT-RF technology is similar in a number of ways to the electromagnetic waves generated by cell phones. Numerous studies in normal humans have shown that such exposure can provide beneficial cognitive changes to the EEG, increase brain energy production, and has no deleterious effects on health over many years. Moreover, recent epidemiologic studies involving over a million subjects have all concluded that electromagnetic exposure to the brain (via cell phones) for 15-20 years does not increase the risk of any form of cancer, including brain gliomas.
Clinical Studies
All of NeuroEM’s clinical studies have administered TEMT-RF to Alzheimer’s patients using our first-of-its-kind and patented medical device, the MemorEM™. This device was deemed a “non-significant risk” device by the Western Institutional Review Board and the FDA has bestowed its first Breakthrough Device designation against Alzheimer’s to the MemorEM device and NeuroEM’s TEMT-RF technology.
The MemorEM device is self-contained and has been designed for in-home daily treatment, allowing for complete mobility and comfort in performing daily activities during treatment (Figure 1A). The device has a custom printed circuit board (control panel) that is powered by a rechargeable battery. This control panel/battery box is worn on the upper arm and wired via a cable to eight (8) uniquely bioengineered emitters embedded between a double-layered head cap worn by the subject. (Figure 1B). The MemorEM device shown below is NeuroEM’s Generation One (Gen1) device used in the company’s already-completed clinical trials. NeuroEM is currently developing newer-generation devices for both commercialization and advanced clinical trials.
Figure 1: The MemorEM™ Head Device
Human head computer simulations performed by NeuroEM show that the 8 emitters collectively provide both global and penetrating TEMT-RF exposure to the human forebrain, including the cerebral cortex and underlying structures (Figure 2).
The figure of computerized simulations shows the electric field penetration from each emitter when on. Importantly, only one emitter is active at any given time, with rapid sequential activation of all 8 antennas over 200 times per second. Similar brain penetration results were seen utilizing a human head phantom in actual electromagnetic lab testing.
Figure 2: TEMT-RF penetration into the brain
No deleterious side effects/behaviors were reported by the subjects or their caregivers during our initial 2-month period of daily TEMT-RF or during a total of 18 months of daily TEMT-RF over a 2½-year period. As well, no significant changes in blood pressure or temperature occurred during treatment sessions, and no abnormalities were observed in post-treatment MRI scans (e.g., no tumors, no new micro-hemorrhages) – all suggesting that global brain TEMT-RF is a safe therapeutic in Alzheimer’s subjects at current parameters and study duration.
Safety
Cognitive/Functional Performance
In an initial Pilot clinical trial involving eight mild/moderate Alzheimer’s subjects, twice daily 1-hour TEMT-RF was provided in-home for two months by caregivers.
Following this treatment period, a reversal of cognitive impairment was observed for 7 of 8 subjects in ADAS-cog and for all 8 subjects in Rey AVLT (Figure 3).
The combination of our extensive pre-clinical studies and this initial clinical study’s cognitive findings resulted in FDA providing Breakthrough Device designation for TEMT-RF technology and the MemorEM device against Alzheimer’s in 2020 — the first drug or device receiving that designation.
Figure 3: TEMT-RF Enhances Memory in ADAS-cog and AVLT
Because of the promising results from the aforementioned initial clinical study, and the enthusiasm of the study’s Alzheimer’s patients/caregivers to continue treatment, 4-month and 12-month extension studies were performed, with breaks of 8- and 5-months interposed with no treatment (31 months total). Despite the two lengthy periods of no treatment, daily in-home TEMT-RF was able to safely stop otherwise certain cognitive decline over a 2½-year period in all six cognitive/functional tasks individually, as well as in an overall “composite” cognitive analysis. Moreover, caregiver assessments throughout that 2½-year period indicate no change in the Global Deterioration Scale (GDS) stage of Alzheimer’s – in other words, the caregivers felt their Alzheimer’s subjects had not declined in cognitive/functional abilities over a 2½-year period. Most non-treated Alzheimer’s subjects would have substantially declined in memory and in their ability to perform daily activities of living over this extended treatment period.
Our clinical cognitive results strongly argue for the initiation of a placebo-controlled, larger clinical trial with TEMT-RF to treat established Alzheimer’s. As such, a Phase IIb/III (Pivotal) clinical trial is anticipated to be initiated soon.
Alzheimer’s Markers in Blood and Brain
Long-term TEMT-RF through 14-27 months induced changes in, or modulated, major Alzheimer’s markers in both cerebrospinal fluid (CSF) and plasma, suggesting that TEMT-RF has effects on the actual Alzheimer’s pathogenic process.
In CSF, long-term TEMT-RF induced reductions in levels of C-reactive protein, p-tau217, Aβ1-40, and Aβ1-42, while modulating CSF oligomeric Aβ levels. In plasma, long-term TEMT-RF modulated/rebalanced levels of both p-tau217 and total tau. These TEMT-RF-induced effects on Alzheimer’s markers were evident many months after the start of treatment and may have contributed to the stoppage of cognitive/functional decline over a 2½-year period in the same Alzheimer’s subjects.
Immune Regulation/Rebalancing in Blood and Brain
Cytokine proteins in the blood and brain are the immune system’s “effectors” and are thus critical for immune system actions. Although there is a balance between pro-inflammatory and anti-inflammatory cytokines in young adulthood and middle age, this balance is lost during later aging. The result is a low, chronic level of brain and body inflammation called “inflamm-aging.” Inasmuch as immune system imbalance and the chronic inflammation it causes are involved in most diseases of aging, a therapeutic intervention that could re-balance cytokines in the blood and brain should be effective for reducing diseases of aging and increasing healthy longevity itself.
Figure 4: As is evident for both plasma IL-17α and IL-18, the response to TEMT-RF is dependent on baseline levels, with TEMT-RF inducing convergence (rebalancing) toward aged normal levels (horizontal dashed line).
In this context, 11 of 12 plasma cytokines in the blood of Alzheimer’s subjects were re-balanced by two months of daily TEMT-RF. Alzheimer’s subjects with lower baseline cytokine levels showed TEMT-RF-induced increases in those cytokines after two months of daily TEMT-RF. By contrast, those subjects with higher baseline cytokine levels in plasma showed treatment-induced decreases in plasma cytokines. This cytokine rebalancing by TEMT-RF resulted in a significant decrease in inflammation in the blood.
In the brain/CSF, TEMT-RF induced a similar rebalancing for seven measurable cytokines, with the direction and extent of changes in individual subjects also being linked to their baseline brain/CSF levels. This cytokine rebalancing was usually associated with a large decrease in brain inflammation, as was the case for the blood.
Our results strongly suggest that daily TEMT-RF to Alzheimer’s subjects for two months can “rebalance” levels for 11 of 12 cytokines in blood and brain, which is associated with a reversal of their cognitive impairment. This rebalancing of so many cytokines, in both brain and systemic compartments, appears to be a remarkable new mechanism of TEMT-RF action that may contribute substantially to its potential to stop or reverse Alzheimer’s and other diseases of aging, as well as to potentially increasing healthy longevity itself.
Brain Imaging Analysis
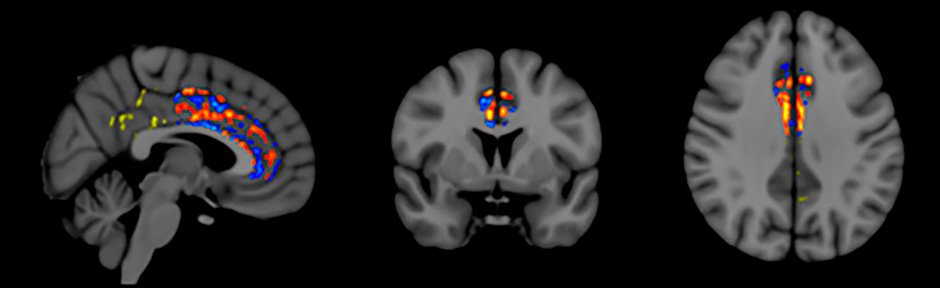
Fractional Anisotropy (FA) is a measure of “functional” brain MRI that is widely used to evaluate functional connectivity (communication) between neurons in the brain. Multiple studies have shown that brain FA consistently decreases as Alzheimer’s progresses (i.e., less neuronal communication). This decrease in FA occurs even over periods as short as three months in Alzheimer’s patients, particularly in a brain area critical for memory integration called the cingulate cortex/cingulum.
Our FA brain imaging focused on the cingulate cortex/cingulum and found that, in addition to the expected regional decreases in FA within this brain area, two months of TEMT-RF resulted in regional enhancements in FA for all subjects.
Figure 5: Increased functional connectivity (red, orange, yellow) in Cingulum following TEMT-RF
Figure 5 shows the FA pattern observed for one subject – red/orange/yellow pixels indicate increased FA following TEMT-RF; blue pixels indicate the expected decrease in FA. These regional FA increases seen in the cingulate cortex/cingulum of Alzheimer’s patients following 2 months of TEMT-RF suggest greater functional connectivity/communication, which may have contributed to the improved memory shown by these Alzheimer’s patients.
Clinical Study Conclusions
TEMT-RF administration to Alzheimer’s subjects was safe while providing cognitive enhancement, changes to Alzheimer’s markers in CSF/blood, immune regulation, and evidence of enhanced brain functionality.
TEMT-RF appears to be disease-modifying against Alzheimer’s, is non-invasive, and is easily administered in-home.
Although these promising results need to be replicated in controlled clinical trials, they suggest that TEMT-RF may provide a vertical leap to an entirely new bioengineering-based intervention against Alzheimer’s disease.
Publications
-
Transcranial Electromagnetic Wave Treatment: A Fountain of Healthy Longevity?
International Journal of Molecular Sciences, 2023, 24, 9652
Arendash, G.; Cao, C. -
Transcranial Electromagnetic Treatment Stops Alzheimer’s Disease Cognitive Decline Over a 2½ Year Period: A Pilot Study
Medicines, Vol. 9, August 3, 2022
Arendash, G., Abulaban, H., Steen, S., Andel, R., Wang, Y., Bai, Y, Baranowski, R., McGarity, J., Scritsmier, L., Lin, X, Shen, N, Aljassabi, A., Li, Y, and C. Cao. -
Transcranial Electromagnetic Treatment “Rebalances” Blood and Brain Cytokine Levels in Alzheimer’s Patients: A New Mechanism for Reversal of Their Cognitive Impairment
Frontiers in Aging Neuroscience 14: Article 829049, 2022
Cao, C., Abulaban, H., Baranowski, R., Wang, Y., Bai, I., Lin, X., Shen, N, Zhang, X., and G. Arendash. -
A Clinical Trial of Transcranial Electromagnetic Treatment in Alzheimer’s Disease: Cognitive Enhancement and Associated Changes in CSF, Blood, and Brain Imaging
Journal of Alzheimer’s Disease, Vol 71: 57-82
Arendash, G., Cao, C., Abulaban, H., Baranowski, R., Wisniewski, G., Becerra, L., Andel, R., Lin, X., Zhang X., Wittwer, D., Moulton, J., Arrington, J., and A. Smith. -
Review of the Evidence that Transcranial Electromagnetic Treatment (TEMT) will be Safe and Effective Against Alzheimer’s Disease
Journal of Alzheimer’s Disease 53: 753-71
Arendash, G.W. -
Transcranial Electromagnetic treatment (TEMT) for Alzheimer’s Disease: Why it has the potential to trump Alzheimer’s drug development
Journal of Alzheimer’s Disease 32:243-266, 2012
Arendash, G.W. -
Long-Term 918 MHz Electromagnetic Field Treatment to Very Old Alzheimer’s Mice Reverses β-Amyloid Deposition, Modifies Regional Cerebral Blood Flow, & Provides Selected Cognitive Enhancement without Brain Hyperthermia
PLoS ONE, 2012
Arendash G.W., Mori, T., Dorsey, M., Gonzalez, R., Tajiri, N., and C. Borlongan. -
Electromagnetic field treatment enhances neuronal activity: Linkage to cognitive benefit and therapeutic implications for Alzheimer’s Disease
Journal of Alzheimer’s Disease and Parkinsonism Vol. 1:102, 2011
Mori, T. and G.W. Arendash. -
Long-term electromagnetic field treatment enhances brain mitochondrial function of both Alzheimer’s transgenic mice and normal mice: A mechanism for electromagnetic field-induced cognitive benefit?
Neuroscience 185: 135-149
Dragicevic, N., Bradshaw, P.C., Mamcarz, M., Lin, X., Wang, L., Cao, C., and G.W. Arendash. -
Electromagnetic Field Treatment Protects Against and Reverses Cognitive Impairment in Alzheimer’s Mice
Journal of Alzheimer’s Disease 19: 191-210, 2010
Arendash., G.W., Sanchez-Ramos. J., Mori, T., Mamcarz, M., Lin, X., Runfeldt, M., Wang, L., Zhang, G., Sava, V., Jun Tan, J., and C. Cao.